OCT as Optical Biopsy in Dermatology
Skin is the largest organ of the body, possessing an amazing ability to heal itself even after considerable damage. As our primary barrier against microbes and the elements, it is key that we understand the dermal repair process and how to speed it. OCT offers a novel and nondestructive method to do so, peering beneath the skin to depths of 1-2 mm, and with better than 10 µm resolution. To prove out this potential, a group led by Irena Pastar at the University of Miami decided to evaluate the use of OCT as an alternative to tissue sectioning in wound healing studies. The result? OCT was able to capture detailed wound images rivaling those collected via microscopy, and provided a more objective assessment of the healing benefits of a new stem cell therapy.
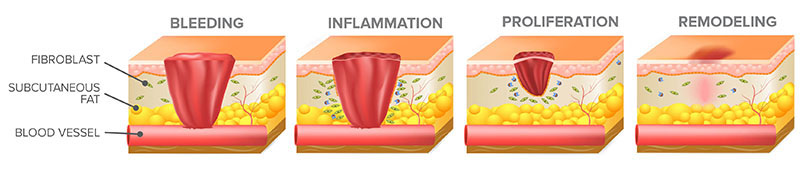
Revealing the healing process
During wound healing, the skin goes through overlapping phases of inflammation, proliferation (or migration), and remodeling. Although the process of acute wound healing isn’t studied in humans in-vivo for ethical reasons, it can be closely mimicked ex-vivo. The ‘ex-vivo’ model for wound healing uses donated skin from reduction surgeries, sustaining it under controlled conditions long enough to study the molecular mechanisms involved, and to evaluate therapies intended to speed healing.
The primary drawback of this model is that assessment has traditionally required destruction of the wounds to evaluate the healing process via histologic examination (HE). Multiple identical wounds must be made to study a single wound type or therapy, carefully sectioning and staining each in turn for examination under a microscope at various time points to see how far the epithelial tongue has grown from the wound edge to cover the exposed dermis. OCT, in contrast, allows the same wound to be monitored non-destructively from start to finish, eliminating variability between tissue samples and individual wounds. This is particularly relevant given that wounds tend to heal unevenly, closing more quickly at some edges than others.
OCT can be used to quickly collect longitudinal images in cross-section that reveal dermal sub-structure, from which 3D volumes or en-face images of the whole wound can be generated. This facilitates a more objective view of the wound as it heals, rather than being limited to a specific cross-section and the quality of sample preparation.
Evaluating OCT for ex-vivo
In this study, both swept-source (SS-OCT) imaging at ~1300 nm and spectral domain (SD-OCT) imaging at ~850 nm were evaluated for use in assessment of cutaneous wound healing. While both systems were able to image to similar depth (~2 mm and ~1.9 mm, respectively), the SD-OCT system from Wasatch Photonics offered greater axial resolution (<3 µm) and transverse resolution (<6 µm) than the clinical SS-OCT system. Shorter operating wavelengths offer higher resolution OCT images, allowing better differentiation of the different tissue types within the dermal structure.
To compare OCT imaging to histologic examination (HE), a series of identical wound samples were prepared, a portion of which were treated with rhEGF (recombinant epidermal growth factor). All were incubated at 37°C, and triplicate samples were collected at days 0, 4, and 7 for evaluation using each technique. Wounds to be assessed by HE were fixed in formalin solution, embedded in paraffin, and then sectioned in 8 µm slices for staining and microscopy, sampling from the edge of the wound to its widest point in diameter. Healing progress was calculated by comparing the sum of the epithelial tongues migrating from the wound edge to the total wound diameter as a percentage.
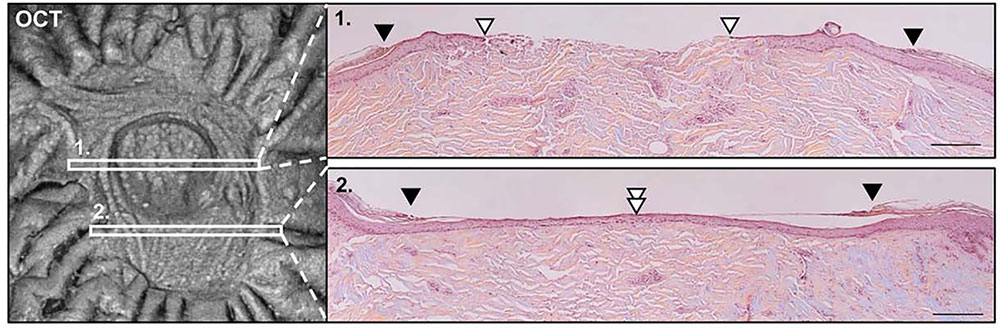
For the OCT analysis, samples could be measured in cross section from one wound edge to the other in <1 minute, building up en-face wound images of the tissue surface from the longitudinal scans. In these ‘top-down’ views of the wound, the intact epidermis could be easily distinguished from the migrating epithelial tongue as it extended to cover the exposed dermis at the center. This allowed a more objective area calculation to be used to quantify healing in terms of percent of wound healed vs the original wound area.
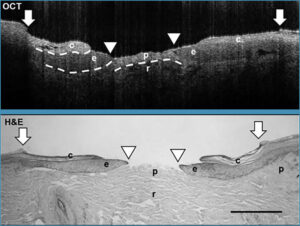
OCT consistently revealed a higher degree of re-epithelialization (healing) as compared with HE analysis, which is not surprising given its ability to image the full wound vs extrapolating from cross-sections. Still, the data showed a strong positive correlation between OCT and HE analysis, and almost perfect agreement for both new and fully healed wounds, validating OCT as a viable technique for analysis.
Cross-sections obtained using the 800 nm SD-OCT system from Wasatch Photonics provided sufficient resolution and contrast to clearly identify the epidermis, the papillary and reticular dermis, and the migrating epithelium in the wound bed. This ability to differentiate between tissue types extends the applicability of OCT in dermatology well beyond evaluation of wound healing or novel therapies to studies of the mechanisms of stem cell action and epidermal migration during re-epithelialization. OCT’s ability to provide rapid, reproducible scans non-invasively holds great promise for use in preclinical testing, and for guiding in-depth immunohistochemistry analyses by identifying the best location for sectioning.
Longitudinal cross-sections acquired using 850 nm SD-OCT at 0, 4, and 7 days postwounding, imaging from one wound edge to the other in ~10 µm increments, collected at 22 frames per second.
A novel stem cell therapy
After validating OCT as a viable ‘optical biopsy’ alternative to histologic analysis, Pastar’s group applied this new imaging modality to analyze the therapeutic potential of allogeneic human adipose derived stem cells (ASC’s) in human ex vivo wounds. Stem cell therapies have shown promise for the treatment of difficult wounds, but their use can be controversial. Stem cells derived from adult adipose (fatty) tissue, however, are free of this constraint, and have been shown to promote various aspects of wound healing, from differentiation to vasculogenesis.
In tandem with the validation study previously described, a series of ex vivo wounds were treated with ASC’s via an injection into the center of the fresh wound. These wounds were also monitored with OCT for a period of 4 days – the exponential phase of healing in ex-vivo wounds. OCT imaging found that all ASC-treated wounds had completely re-epithelialized, while media treated control wounds showed 50% wound closure. The difference in healing for the ASC-treated wounds vs rhEGF-treated wounds was found to be statistically significant, and the enhancement of wound closure was confirmed via cross-sectional OCT images identifying both single layer and differentiated layers of epidermis. the ability of allogeneic human ASC to promote re-epithelialization. For 3 mm diameter wounds extending to the dermis, a single ASC treatment resulted in significant stimulation of healing, documented by OCT. Given the track record of the ex-vivo wound model for evaluating novel therapeutics, and the ability of OCT to be used in preclinical studies, the evaluation of ASC therapy with OCT for in-vivo use in wound treatment holds great potential.
Conclusion
By imaging ex-vivo wounds non-destructively to 2 mm depths and better than 10 µm resolution, OCT has demonstrated both its feasibility and superiority for noninvasive monitoring of human wound healing. Not only is it faster and less variable than histologic examination, but it also permits continuous monitoring throughout the wound healing process and sufficient resolution to assess both anatomy and pathology. This makes it a promising technique for applications in wound healing and the evaluation of novel therapeutics in both laboratory and clinical settings. When used to study treatment of wounds with ASC’s, OCT revealed a significant increase in re-epithelialization after just 4 days, further proving OCT’s potential to make a significant contribution to the field of wound healing.
Want to talk more about using OCT for your application in dermatology or other tissue? Contact us
References
Glinos, George D., et al. “Optical coherence tomography for assessment of epithelialization in a human ex vivo wound model.” Wound Repair and Regeneration 25.6 (2017): 1017-1026.


